Organic Cluster Apparatus(Orcla)
Researching PhD student: Kevin Schwarz
Most recent publications: Anion Photoelectron Spectroscopy of Acenes
Introduction and motivation
Understanding the electronic properties of organic molecular solids attracted a lot of interest, because of their vast applications in science and technology [1]. The molecular clusters of organic materials can offer an excellent model to study the evolution of electronic properties from single molecule to bulk solid. Here the construction of a new source for molecular organic clusters is presented. The important aspect of this source is the high-temperature pulsed valve which has the operating temperature of over 500°C.
Generation and study of mass-selected organic clusters
Molecular clusters are generally produced by supersonic expansion of diluted molecules in a carrier gas [2]. This process supplies enough cooling for the generation of molecular clusters. The efficient large molecule cluster generation in this method needs high pressure of seeded molecule and carrier gas. So, the pulsed valve which is used in this kind of experiment should work at high pressure and temperature [2]. In the expansion region, the clusters get ionized. The negatively ionized clusters are guided by a skimmer to the next chamber, which is a TOF mass spectrometer for size selection. The mass-selected clusters are guided to the interaction region of the photoelectron spectrometer. In this region, the interaction of the laser light and negatively ionized clusters leads to the generation of free electrons. The kinetic energy of these electrons will be measured in the magnetic bottle-type photoelectron spectrometer. Fig. (1) illustrates all these processes clearly. The obtained spectrum gives an insight into the evolution of the electronic structure of the clusters as they grow.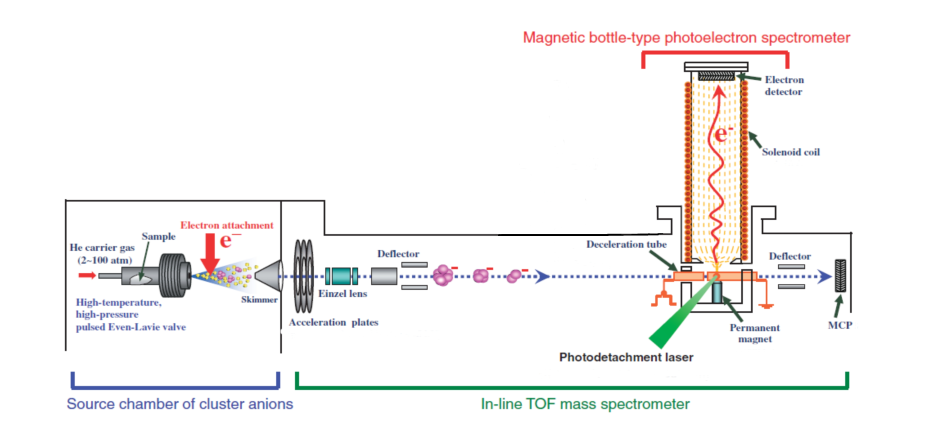
Molecular cluster Sources
Common sources for molecular cluster generation can be divided in three major categories.
a) Knudsen (effusive) Sources
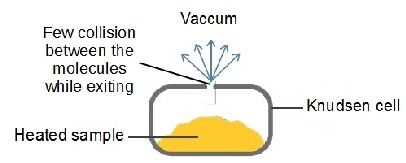
- Sample is heated. The evaporated molecules leave the cell. This effusion is happen in thermodynamic equilibrium.
- The mean free path of the evaporated molecules is larger than the hole size.
- Drawback:low intensity of the clusters.
b) Aggregation Sources
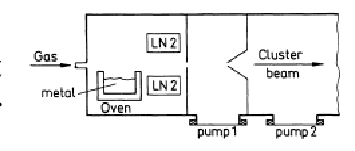
- Evaporated molecules of the sampled are cooled by a colder gas. It helps the molecules to condense and clusters are generated.
- In comparison to Knusden source it provides more intense and bigger clusters
- Drawback: The problem of reproducibility.
c) Supersonic cluster source
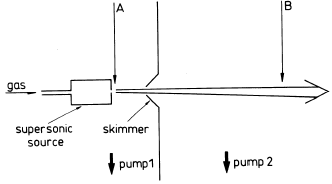
- Expansion of the high-pressure gas through a small hole. Molecules make lots of collisions during the expansion. Adiabatic cooling happens. This very cold environment leads to the condensation of molecules and cluster generation.
- It is the preferred method for the generation of molecular clusters.
New supersonic molecular cluster source
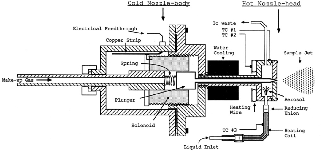

- Organic clusters are molecular clusters and their preferred method of generation is supersonic expansion. The essential component for this purpose is a high-temperature pulsed valve. The maximum operating temperature of the commercially available high-temperature pulsed valves (300°C) is much less than our required temperature (600°C). So, a new high-temperature pulsed valve was designed and constructed.
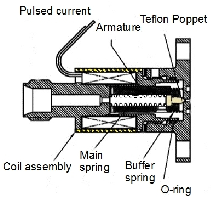
- This new design combines the idea of Li & Lubman with the solenoid of a General-Parker pulsed valve as an actuator.
- In the design of Li & Lubman, all the parts are made of metal so the temperature of the valve head can be increased a lot. In fact, the Teflon poppet and viton o-ring limit the maximum operating temperature of the General-Parker valve. The other aspect of the Li & Lubman design is the distance between the heated head of the valve and the actuator which let the actuator be cooled by water and protects it from the high-temperature of the nozzle.
- The drawback of Li & Lubman design is the bad metal-metal sealing of the nozzle. We removed this drawback by using a hard metal (steel) and a soft metal (copper). This modification works well and our valve has very good sealing.
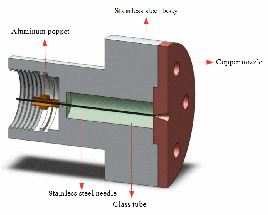

- In this new design, the solenoid of the General-Parker pulsed valve is used as the actuator.
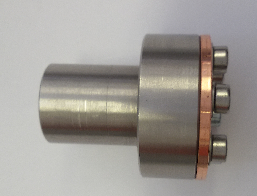
- The head of the valve is made of steel and the nozzle is made of copper. The hole in the nozzle (D=0,1 mm) is closed by a sharp tattoo needle (L=30mm, D=0,35 mm) which is made of steel. The movement of the needle is guided by a glass tube (ID=0,375 mm).
- Because the needle is made of steel and the nozzle is made of a softer metal (copper), the sealing gets better and better by working the valve.
- After well adjusting the valve and becoming sure about its operation by measuring the gas output when the valve is off and on, it is fixed in the source chamber. A simple holder was constructed and the valve was fixed on an item bar in front of the skimmer.
- The ionization of the gas beam is done by an electron gun.
- To find if the expansion of the valve is good enough to generate cluster, CO2 is used as the inlet gas in order to produce CO2 Clusters.
- The obtained mass spectrum of the generated clusters is compared with the spectrum reported by the other groups. We also observed the magic numbers 14 and 16 in our spectrum.
- This experiment shows the good gas expansion which is needed for cluster generation. It also proved the stable operation of the valve.
Conclusion and outlook
In this project, a new cluster source is being constructed in order to generate and study organic clusters. The essential requirement for this purpose is a high-temperature pulsed valve with an operating temperature over 500°C. The constructed valve works very well and CO2 clusters were generated by that at room temperature. The design has the capability to increase the temperature. The high-temperature version of the valve has been designed and it is under construction.- N. Ando, M. Mitsui, J. Chem. Phys. 127, 234305 (2007)
- M. Mitsui, A. Nakajima, Bull. Chem. Soc. Jpn. 80, 6 (2007).
- www.azom.com/equipment-details.aspx?EquipID=3430 (18,07,2018).
- H. Haberland, Clusters of Atoms and Molecules (Theory, Experiment, and Clusters of Atoms); Springer-Verlag, 1994.
- A. P. L. Wang and L. Li, Anal. Chem. 64, 7, (1992).
- General pulsed valve manual.
- M. Knapp, D. Kreisle, O. Echt, K. Sattler and E. Recknagel, Surface Science 156, 313 (1985).
