Big Experiment for Research on Temperature controlled cluster Anions and cations (Berta)
Researcher : Lukas Weise
Most recent publications: Angle-resolved photoelectron spectroscopy of size-selected coinage metal cluster anions and Decoherence-Induced Universality in Simple Metal Cluster Photoelectron Angular Distributions
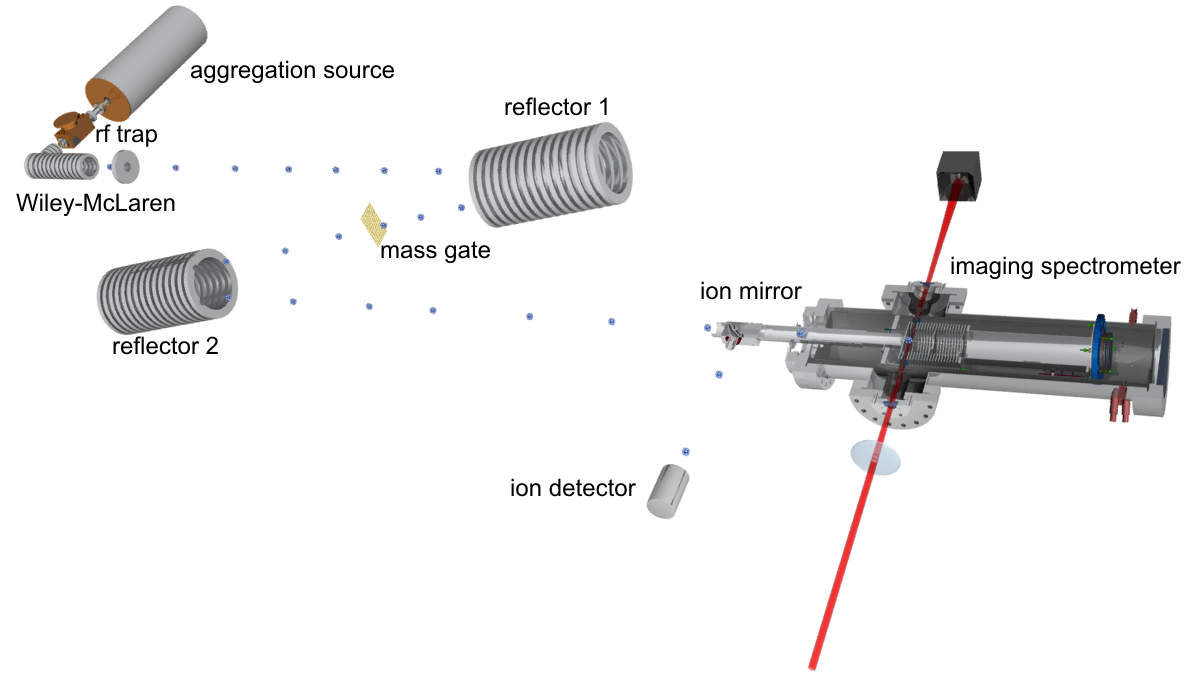
The total setup
Sodium and aluminum clusters were produced in an evaporation respectively magnetron sputter source. Sodium atoms were evaporated from an oven, condensed to clusters in a liquid-nitrogen-cooled He gas flow, and ionized by an electric discharge.
Experimental results for Sodium clusters
Observations
- For all investigated cluster sizes the β parameters show a smooth evolution with the excitation energy.
- Especially for bigger sizes β reaches strongly negative values. This can only occur by the interference of two outgoing partial waves which leads to the assumption of a high coherence during the excitation process, even for very big many-particle systems such as Na147-.
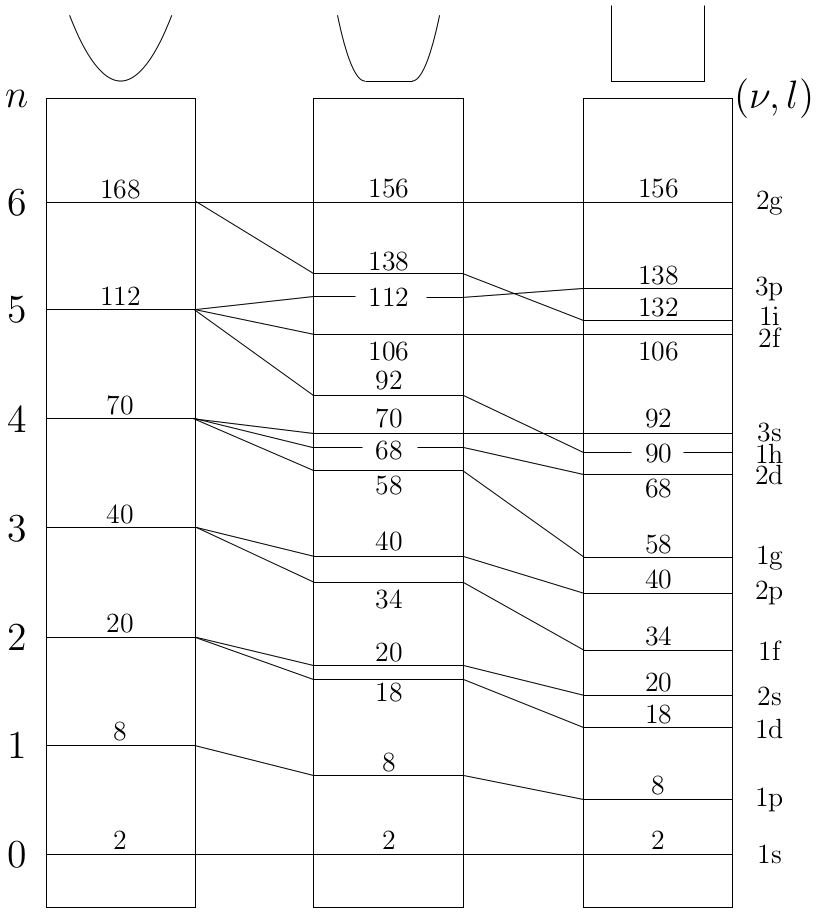
Comparison of the approximate angular momentum eigenstates for different cluster sizes
- Characteristic differences exist for the evolution of β parameters of jellium states with different l quantum numbers.
- Energetically split jellium states from the same cluster size show a very similar evolution of β if plotted versus the kinetic energy of the detached electron.
- Another similarity is observed between β parameters for jellium states with the same l quantum number but from different cluster sizes.
→ The quantum number l still satisfyingly characterizes the bound states even for nonspherical symmetries.
Theoretical background
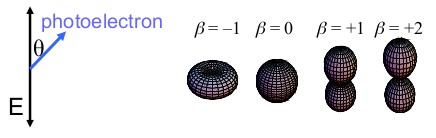
Data of Ag55 and Cu55 clusters and comparing with Na55
- Splitting of 1g and 1f level because of icosahedral structure.
- In contrast to sodium and copper both outer peaks of silver don't overlay.
- DFT calculations show very similar electron densities for silver and sodium.
- Peaks of the 1g level: compared to sodium the minima of the distributions of copper and silver are shifted to higher kinetic energies.
- Peak of the 2p level: the distributions of copper and silver are very similar.
- Minima of the Betaparameter distributions are similar when plotted against the kinetic energy multiplied by the clustersize.
